Digital. Connected. Reliable.
Your Partner for Digital Labs and Connected Research
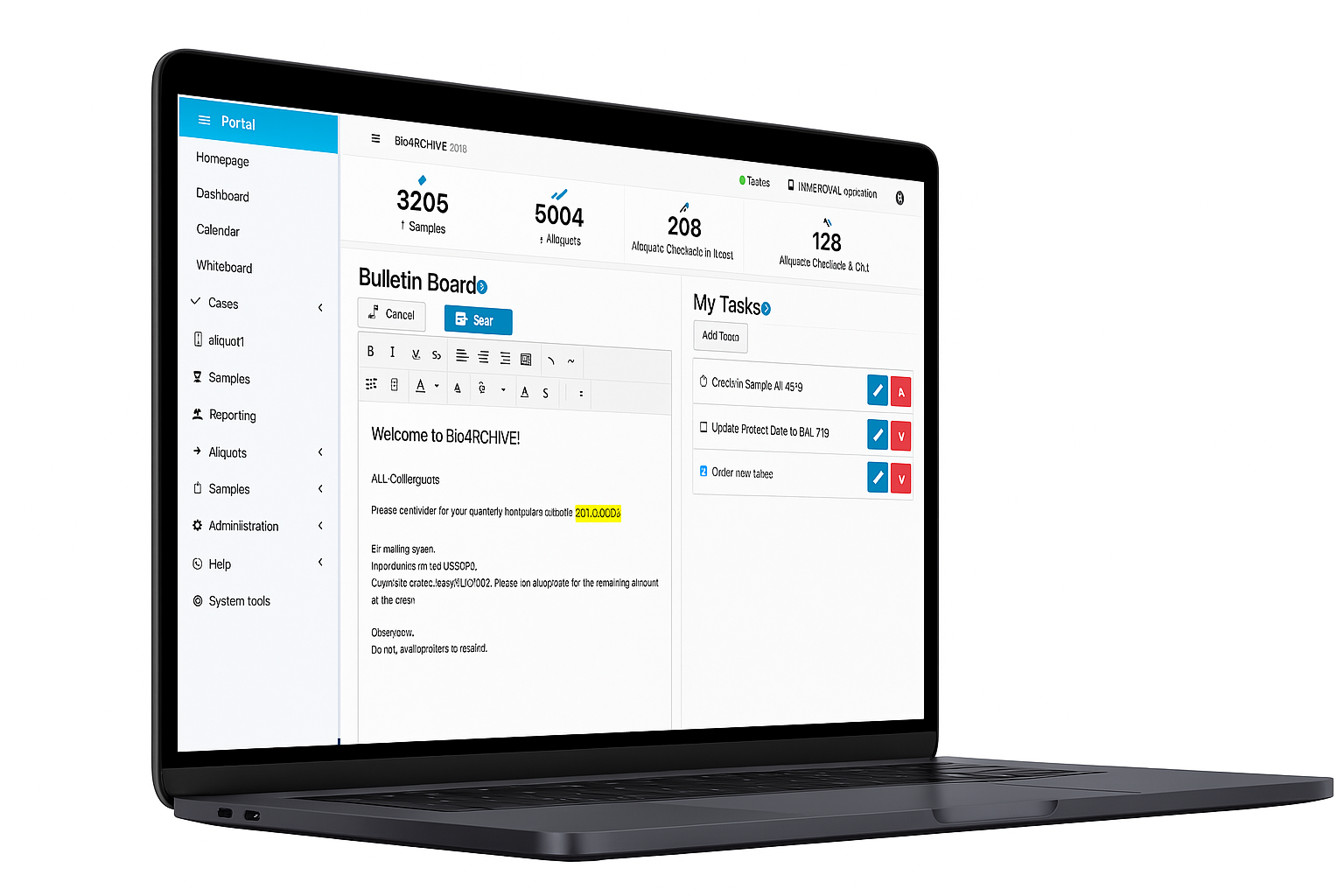
We offer you a freely configurable all-in-one platform for managing your biobank, your electronic laboratory notebooks and your studies.
With our “BioARCHIVE” software, you can reliably track samples, efficiently manage experiments and studies, and centrally organise inventory and teams in one system.
- Flexible & customizable: Your workflows are unique – and so is our software. BioARCHIVE adapts dynamically to your processes and data structures.
- Simple & intuitive: Clear masks, sophisticated functions and intuitive operation save valuable time in everyday work.
- Safe & compliant: With BioARCHIVE you meet the highest standards – GDPR, EU GMP Annex 11 and FDA 21 CFR Part 11 included.
- Transparent & fair: No hidden costs: you only pay for concurrent users. Updates, support and maintenance – all included.

Flexible documentation
Customizable & efficient. Configure individual input masks, integrate relevant links and adapt everything dynamically to studies, cases or work areas.
Intuitive documentation & clear interface. Structure data and lists clearly. Conditional field displays ensure a tidy, user-friendly view.
Safe & team-oriented. Assign precise access rights, enable collaborative working and secure critical data with dual signatures.
Sample management
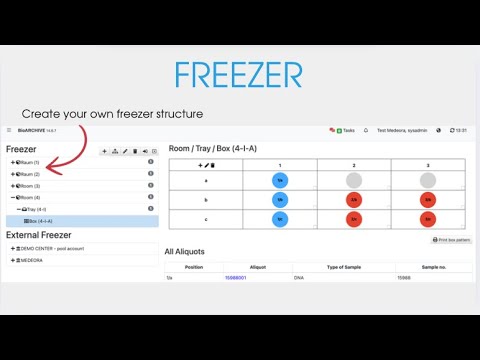
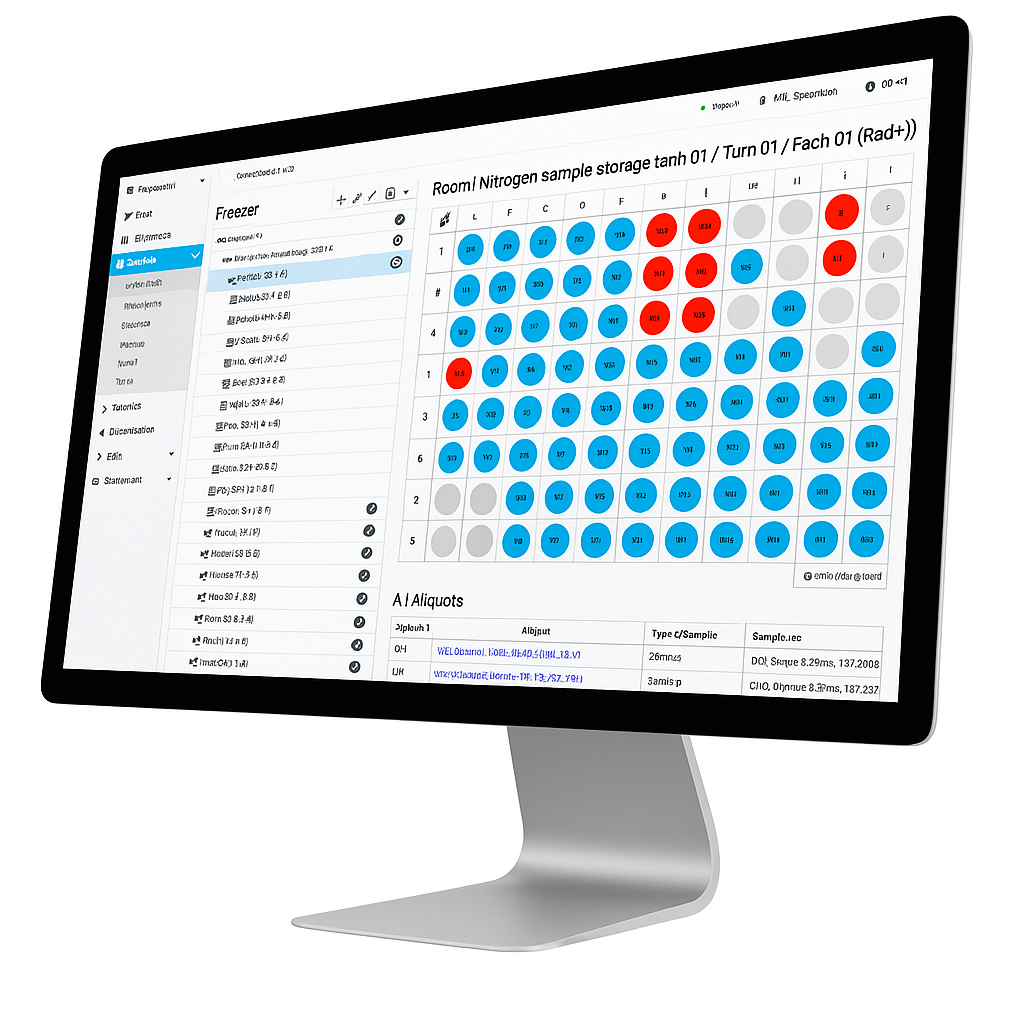
Intuitive & structured. Record, import and manage samples quickly and precisely – with visual storage locations, clear suffixes and powerful filter functions.
Complete documentation. Link images, reports and relationships directly to your samples. Every change remains traceable – archived, not deleted.
Flexible & team player. Manage storage locations dynamically, reserve samples early and work efficiently as a team – with a full overview and targeted approvals – even together with other work groups.
Team organization
Clearly prioritized tasks. Everyone in the team keeps their focus with individual to-do lists – structured, clear and targeted.
Transparent progress monitoring. Use meaningful metrics to analyze performance and progress in real time – for well-founded decisions.
Smooth cooperation. A central bulletin board and integrated management data collection promote information flow, organization and efficiency throughout the team.
Electronic Laboratory Notebook (ELN)
Flexible & intuitive. Keep as many lab notebooks as you like, create entries with text, tables, images or documents – simply using the editor or drag & drop.
Networked data & easy navigation. Link relevant samples or studies directly in the entry. Find everything again – thanks to tags, full text search and tree structure.
Confident & team player. Share entries in a team, set electronic signatures and meet all compliance requirements such as GxP, FDA 21 CFR Part 11 and GDPR.
Integration & Quality
Seamless integration & flexible data formats. Import and export data in common formats such as CSV, XML, Excel, Word and PDF – including barcode and rack scanner support from leading manufacturers.
Maximum security & transparency. Use role-based access rights, dual signatures and seamless audit trails to ensure data integrity and traceability.
Regulatory safeguards & continuous monitoring. Complies with GDPR, EU GMP Annex 11 and FDA 21 CFR Part 11 – supported by an internal monitoring system to ensure documentation quality.
Budget
Fair & scalable. Pay only for simultaneously active users – register as many as you like and flexibly adjust your licenses at any time.
100 % cost transparency. No additional costs for support, updates or services – everything is included in the price.
Efficiency meets economy. Optimize your usage, retain full control over your expenditure and benefit from a licensing model that grows with you.