MEDEORA
NEWS
MEDEORA
news
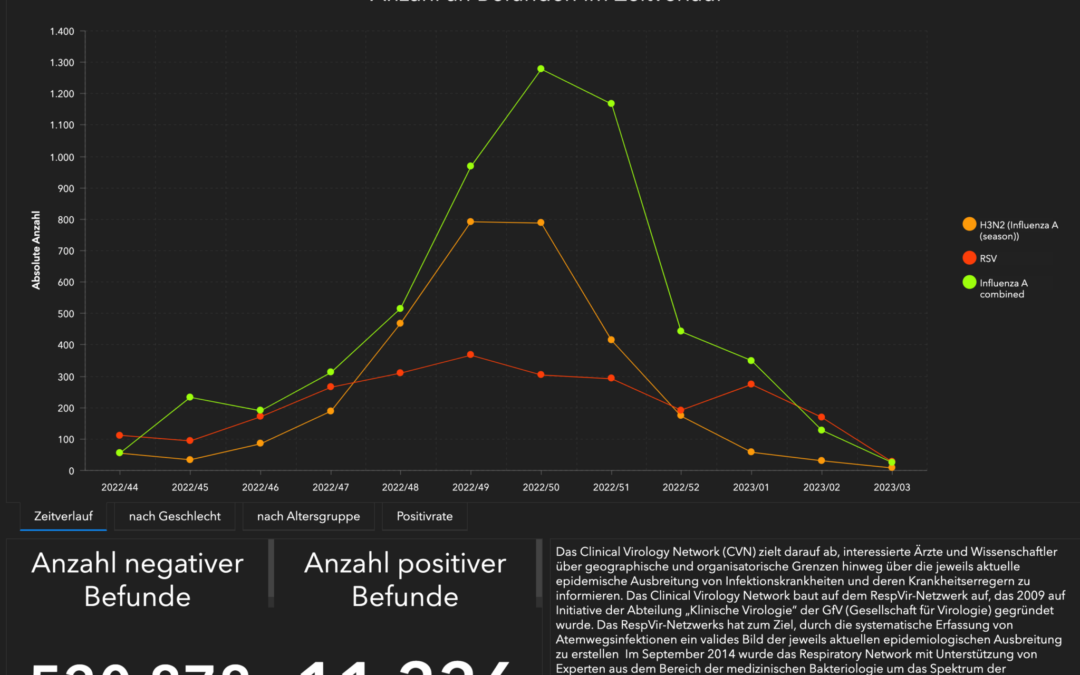
MEDEORA supports the “Clinical Virology Network (CVN)” again this year
The Clinical Virology Network (https://public.clinical-virology.net) aims to inform interested physicians and scientists across geographical and organizational boundaries about the current epidemic...

Research activities of MEDEORA GmbH
We are pleased to announce that the technical article on respiratory viruses, to which our managing director Dr. Norbert Schmeißer contributed significantly, has now been published. The article...
Marketing-Relaunch
We have used the year 2021 to reorganize our product range.So it seemed right and important to us to redesign the logo and the websites.These changes have been incorporated into our new website.The...

Upcoming changes at MEDEORA GmbH
Dear customers, supporters and interested parties, Today we would like to inform you in advance about the upcoming innovations at MEDEORA. On 12 December 2012, Dr Norbert Schmeißer took over MEDEORA...

BioRiver: IT-supported stability testing
Date - Home BioRiver Events Dear people responsible for quality control, clinical research and IT, We would like to invite you to an exchange of experiences in the IT working group of BioRiver eV!...

MEDEORA not at the LIMS Forum Online this year
There is no substitute for personal contact! The LIMS Forum has usually been a fixed date in our calendar for several years. It was therefore all the more difficult for us to forego our presence...
NEWS
